Synthesis of 1,4-Diketones from Esters Enabled by a Tetraborylethane Reagent
Miaomiao Wu1,2, Tongchang Fang2, Liangxuan Xu2, Qingfeng Xu1(徐庆锋)*,Jianmei Lu1(路建美)*,Chao Liu2,3(刘超)*
1College of Chemistry, Chemical Engineering and Materials Science, Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu 215123, China
2College of Chemistry, Chemical Engineeringand Materials Science, Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu 215123, China;
3State Key Laboratory of Coordination Chemistry, Institute of Green Chemistry and Engineering, School of Chemistry and Chemical Engineering, Nanjing University,Suzhou, Jiangsu 215163, China
Org. Lett. 2025, 27, 1175−1180
Abstract: A modular synthesis method for 1,4-diketones has been developed. Utilizing inexpensive carboxylic acid esters as carbonyl sources and tetraborylethane (TBE) as a nucleophilic reagent, a one-pot strategy for constructing two C–C bonds was established. Notably, this reaction proceeds without the involvement of transition metals and exhibits excellent functional group compatibility. A diverse array of α-substituted 1,4-diketones were synthesized using various electrophiles for capture.
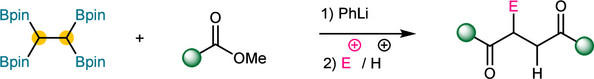
Article information: //doi.org/10.1021/acs.orglett.4c04690