Visible-Light-Induced Annulation of Benzothioamides with Sulfoxonium Ylides To Construct Thiazole Derivatives
Huangchu Lin, Yuzhu Peng, Xiaoguang Bao(鲍晓光)*
Innovation Center for Chemical Sciences,College of Chemistry, Chemical Engineering and MaterialsScience, Soochow University, Suzhou, Jiangsu 215123, China, Jiangsu Key Laboratory of Advanced Negative CarbonTechnologies, Soochow University, Suzhou, Jiangsu 215123
Org. Lett. 2025, 27, 629−634
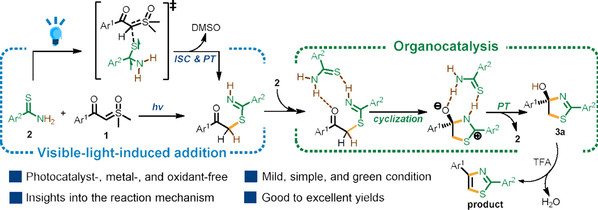
Abstract: Herein, visible-light-induced annulation of benzothioamides with sulfoxonium ylides to furnish thiazole derivatives isdeveloped under transition-metal-, photocatalyst-, and oxidant-free conditions. This protocol exhibits good substrate scope, affordingthe desired products with satisfied yields in a mild and green manner. Detailed mechanistic studies suggest that the benzothioamidesubstrate plays a dual role in this reaction. Under visible-light irradiation, excited benzothioamide, in its triplet state, could undergo Sattack to the C=S moiety of the sulfoxonium ylide followed by the dissociation of dimethyl sulfoxide and H migration to give a keyadduct. In addition, benzothioamide could act as an organocatalyst to facilitate the intramolecular cyclization of the adduct andproton transfer steps. After the dehydration of the cyclized intermediate, the desired thiazole derivatives can be produced.
Article information: //doi.org/10.1021/acs.orglett.4c04431