Enantioselective Discrimination of l-Phenylglycine Ethyl Ester through Supramolecular Assembly with a Binaphthyl-Linked Zinc Bisporphyrinate Host
Wenjing Liu, Sasa Hao, Fangfang Fu, Jiaxun Jiang, Yue Chen, Chuanjiang Hu(胡传江)*
College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China
Inorg. Chem.2025, 64, 6403–6407
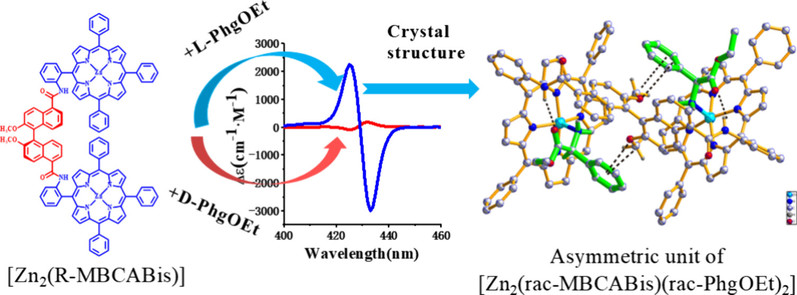
Abstract: Chiral discrimination of amino acid stereoisomers is essential for biological processes and pharmaceutical development. Bisporphyrins represent promising candidates for the chiral recognition of amino acid derivatives. Herein, we report a rationally designed binaphthyl-linked zinc bisporphyrinate complex demonstrating remarkable enantioselective binding toward l-phenylglycine ethyl ester (L-PhgOEt). The complex demonstrates a 19-fold amplification in circular dichroism (CD) response intensity for L-PhgOEt over its d-enantiomer, with quantified enantioselectivity (L/D = 4.8), reflecting good chiral recognition capabilities. Single-crystal X-ray analysis unveils a multimodal recognition mechanism involving (i) axial coordination to zinc centers, (ii) hydrogen bonding interactions with amide functionalities, and (iii) π-system stabilization through both C–H···π and O···π interactions with the methoxy-functionalized binaphthyl linker. This synergistic combination of coordination bonds and noncovalent interactions establishes bisporphyrins as tailorable chiral receptors and guides the design of biomimetic systems for pharmaceutical applications.
Article information: //doi.org/10.1021/acs.inorgchem.5c00479